A_User
#2
@CronosTempi relatively good news for glitazones? I know you like pioglitazone. I haven’t read it yet, though.
Mice bred in 2021 were tested by the Interventions Testing Program (ITP) for possible lifespan benefits of 2BAct (2BA), dichloroacetate (DCA), Epicatechin (EPI), Forskolin (FSK), Halofuginone (HAL) and Mitoglitazone (MIT). All agents were administered in the diet ad libitum beginning at 7 months of age. In male mice, EPI increased median lifespan by ~ 5%, and HAL and MIT each increased median lifespan by ~ 9%. EPI and HAL, but not MIT, increased 90% survival. In addition to adding 3 new agents to the list of interventions identified by the ITP that extend lifespan, this report continues the strong male bias in the efficacy of life-extending drugs identified so far.
Full paper here: Extension of lifespan by epicatechin, halofuginone and mitoglitazone in male but not female genetically heterogeneous mice | GeroScience
3 Likes
Yes, at first glance it might seem so. However, as in the case of canagliflozin ITP success, the question becomes whether the same applies to other SGLT2i, and even possibly those others may work better in humans vs mice. So what I am trying to determine are the differences between mitoglitazone and pioglitazone. Preliminarily, the difference seem fairly pronounced (bigger than between the various SGLT2i). Mitoglitazone is fairly newish, so there’s relatively less data on it in humans vs pioglitazone which is pretty old.
1 Like
Pubmed has like 3 papers on mitoglitazone, other than the ITP result. Pretty thin on the ground.
Here’s a Chinese paper in mice, comparing mitoglitazone and pioglitazone in the context of kidney tissue damage in kidney transplants.
Mitoglitazone ameliorates renal ischemia/reperfusion injury by inhibiting ferroptosis via targeting mitoNEET
“Ischemia/reperfusion- (I/R-) induced injury is unavoidable and a major risk factor for graft failure and acute rejection following kidney transplantation. However, few effective interventions are available to improve the outcome due to the complicated mechanisms and lack of appropriate therapeutic targets. Hence, this research aimed to explore the effect of the thiazolidinedione (TZD) compounds on I/R-induced kidney damage. One of the main causes of renal I/R injury is the ferroptosis of renal tubular cells. In this study, compared with the antidiabetic TZD pioglitazone (PGZ), we found its derivative mitoglitazone (MGZ) exerted significantly inhibitory effects on erastin-induced ferroptosis by suppressing mitochondrial membrane potential hyperpolarization and lipid ROS production in HEK293 cells. Moreover, MGZ pretreatment remarkably alleviated I/R-induced renal damages by inhibiting cell death and inflammation, upregulating the expression of glutathione peroxidase 4 (GPX4), and reducing iron-related lipid peroxidation in C57BL/6 N mice. Additionally, MGZ exhibited excellent protection against I/R-induced mitochondrial dysfunction by restoring ATP production, mitochondrial DNA copy numbers, and mitochondrial morphology in kidney tissues. Mechanistically, molecular docking and surface plasmon resonance experiments demonstrated that MGZ exhibited a high binding affinity with the mitochondrial outer membrane protein mitoNEET. Collectively, our findings indicated the renal protective effect of MGZ was closely linked to regulating the mitoNEET-mediated ferroptosis pathway, thus offering potential therapeutic strategies for ameliorating I/R injuries.”
Epicatechin content in food:
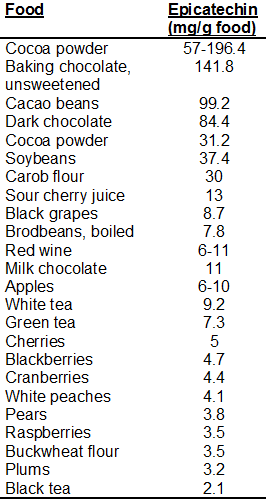
I thought tea had a lot more. It seems cocoa is the way to go, the problem is the amount of heavy metals in most of the brands tested so far. To my fellow Europeans, have you found any good source?
5 Likes
Look here: Low Cadmium Extreme Dark 100 Schokolade - Edelmond Chocolatiers GmbH, 8,75 € - they have different products, specifically tested for cadmium and heavy metals.
2 Likes
FWIW, in October 2023 Consumer Reports conducted tests of chocolates and powders in the US, and the best (lowest levels of lead and cadmium) was Navitas Organics Organic Cacao Powder:
https://www.consumerreports.org/health/food-safety/a-third-of-chocolate-products-are-high-in-heavy-metals-a4844566398/
I have been using Navitas Organics cacao powder for years. Initially, I put a teaspoon of it with the ground coffee in the paper filter, precisely because I was trying to avoid heavy metals contamination. Studies have shown, that similar to coffee, beneficial polyphenols from the coffee and cacao pass through the paper filter, but heavy metals remain mostly in the filter which you then discard. I still do that, but these days, I also add straight Navitas cacao powder to my coffee, stir and drink that way. I did this based on these CR reports, the increasing evidence that cacao polyphenols are substantial contributors to overall health, and my now more advanced age. As I explained in another post, you have to adjust your health regimen to your age. At a more advanced age, the relative importance of avoiding certain risks falls sharply - that includes avoiding pollution and cancer causing chemicals in food. If you have managed to keep those risks low throughout your lifetime by avoiding them, then you can allow the risk to go up in your later years (I’m 67), because it takes time, as in decades for cancer and other negative consequences of pollutants to develop and accumulate. At 67, I’ll be dead before that happens. The calculus is obviously different if you are younger, because there those negative consequences have time to accumulate and cut your life short. So the calculus of risks versus benefits changes. The benefits of cacao outweigh the risks at my age. This was not the case when younger, because for example the cardiovascular benefits of cacao polyphenols for a young heart are tiny, but risk of heavy metals accumulation are great. Now it’s the opposite. Hence, I brutally add a heaping spoonful of cacao powder to my coffee, contemptuous of the heavy metals, though I also hypocritically choose the least polluted organic powder (Navitas). What can I say, I’m a swine.
3 Likes
Another plug for cacao:
Effects of 2-year cocoa extract supplementation on inflammaging biomarkers in older US adults: findings from the COcoa Supplement and Multivitamin Outcomes Study randomised clinical trial
https://academic.oup.com/ageing/article-abstract/54/9/afaf269/8253933?redirectedFrom=fulltext&login=false
2 Likes
LukeMV
#9
I consume mountains of dark chocolate and cocoa powder so that’s good.
As far the others go, cool results. I just wish we got more results for things many of us routinely take (taurine, creatine, etc.). Just have to be a little more patient.
FWIW: I have been a heavy consumer of chocolate for my entire life without regard to brand. I ate any chocolate that tasted good. Now I consume mainly dark chocolate exclusively, not because it is better but because my tastes have changed. I haven’t put Navitas cacao powder in my coffee yet, but maybe I will give it a try. IMO: The health hazards of heavy metals in chocolate have been overblown.
In my mid-80s, my mind is functioning quite well, and I don’t have cancer.
1 Like
For those interested, if minimizing heavy metals is your priority, prefer cacao/cocoa powders sourced from West Africa or labeled “West African origin” (or blends tilted that way). On average they’re lower in cadmium. Unfortunately, Navatos cacao powder is not in this group.
Back of the Navatos cacao powder that I have.
A better choice would be Cacao Powder By Juka’s Organic Co. (Non-GMO, Gluten-Free, Unrefined, Authentic, All Natural From West Africa) 16oz (16)
Brand: Juka’s Organic Co.
4.7 4.7 out of 5 stars (21) Available from Amazon, and it is slightly cheaper.
[image]
adssx
#12
Mitoglitazone = MSDC-160 ( https://www.nia.nih.gov/research/dab/interventions-testing-program-itp/supported-interventions ). It’s not even approved. And after that, the ITP decided to test pioglitazone. I wonder why they chose mitoglitazone in the first place, but they were right anyway.
1 Like
Yes, the ITP folks seem to make choices - or the folks who submit the requests - based on some pretty good knowledge. That makes me wonder all the more about which specific drugs they pick - like cana and not dapa or empa, and mito and not pio. With empa/dapa and pio being so much more popular and widely used, they had to undertake a very specific choice to forego them in favor of the less common. In a way that’s somewhat deflating for those of us who use the more common drugs, lol.
1 Like
adssx
#14
They picked pioglitazone (link above).
Wow, you’re right. Can’t wait for the results. It’s been 2+ years, so likely soon.
I’m just learning about this drug, looks interesting.
Here is a summary of the commercial history of pioglitazone — key patent milestones, regulatory approvals, launches, genericization, safety/regulatory changes, and current status.
Origins & Patenting
- Pioglitazone is a thiazolidinedione class drug, developed by Takeda and co-developed with Eli Lilly in some markets. (PMC)
- It was patented in 1985. (Wikipedia)
Regulatory Approval & Market Launch
- In the United States, the Food and Drug Administration (FDA) approved Actos (pioglitazone hydrochloride) on July 15, 1999 for the treatment of type 2 diabetes mellitus. (FDA Access Data)
- In Europe, approval through the European Medicines Agency (EMA) came about a year later, on October 13, 2000. (PMC)
- Its launched brand name in the U.S. is Actos. (Wikipedia)
Commercial Performance / Sales
- Early expectations: when it was launched, pioglitazone was seen as a safer thiazolidinedione than its predecessor drugs (such as troglitazone, withdrawn for liver toxicity), with hopes of considerable sales. (WIRED)
- It in fact became a blockbuster drug; at various points, its sales were in the billions per year. For example, in 2008, it was among the top-10 drugs by revenue in the U.S. with sales exceeding US$2.4 billion. (Wikipedia)
Genericization
- The first generic versions of Actos were approved in the U.S. on August 17, 2012. (Medscape)
- Multiple manufacturers now supply generic pioglitazone tablets (15 mg, 30 mg, 45 mg) in the U.S. (Drugs.com)
Combination Products
- Pioglitazone has been combined with other agents to broaden its therapeutic use; for example: • ACTOplus Met (pioglitazone + metformin) was approved by FDA on August 29, 2005. (Drugs.com) • Duetact (pioglitazone + glimepiride) approval came in the U.S. around 2006. (Wikipedia) • Oseni (pioglitazone + alogliptin) is another fixed-dose combination with FDA approval in 2013. (Drugs.com)
Safety, Regulatory Concerns, and Withdrawals / Market Limitations
- Over time, safety concerns emerged, particularly regarding risks such as bladder cancer, heart failure, weight gain, edema, etc. Some markets (France, Germany) suspended or withdrew Actos in 2011 over cancer risk concerns. (Wikipedia)
- Regulatory labeling was updated to reflect such risks. (Wikipedia)
Current Status
- Pioglitazone remains an approved, generic drug in many countries, widely prescribed for type 2 diabetes as an insulin sensitizer. (Wikipedia)
- It is no longer under brand-exclusive patent (in many markets), so generic competition has lowered price and broadened access. (Drugs.com)
A_User
#17
Two threads about it:
Ralph DeFronzo interview about it on Peter Attia’s podcast was interesting.
3 Likes
medaura
#19
It’s very interesting, the consistent male bias of favorable interventions, at least in lower life forms. In humans my bet is that rapamycin and estrogen will be the biggest levers for women. These other things might be hit or miss, but the decline in estrogen seems to set off the master cascade of decline for women and replenishing it seamlessly seems to set things right — even helps men. Rapamycin among the organism wide changes also seems to be an anti fibrotic and good at particularly rejuvenating ovaries so that it also delays the orchestrated obsolescence.