What is the conversion rate of Maverick points to USD?
Attia is super smart who is extremely knowledgeable about a wide swath of scientific literature pertaining to lifespan (the shear breadth and depth of his podcasts is truly amazing), and excels at extracting significance/confounders from studies (he’s an engineer  ), so it might be his podcast comments are not of sufficient granularity/context to your detailed “absorption kinetics” question. I would be very surprised if he didn’t understand fundamental nutritional/muscle protein breakdown/synthesis physiology. At the same time, he’s a highly compensated concierge medical practitioner, and isn’t going to put all his patients on a low calorie, low animal protein, intermittent fasting diet (full Roy Wolford) that will have massive pushback and low compliance, even IF he privately believes it’s a superior longevity diet. He’s conflicted.
), so it might be his podcast comments are not of sufficient granularity/context to your detailed “absorption kinetics” question. I would be very surprised if he didn’t understand fundamental nutritional/muscle protein breakdown/synthesis physiology. At the same time, he’s a highly compensated concierge medical practitioner, and isn’t going to put all his patients on a low calorie, low animal protein, intermittent fasting diet (full Roy Wolford) that will have massive pushback and low compliance, even IF he privately believes it’s a superior longevity diet. He’s conflicted.
Re Attia, are you referring to his bias towards high animal protein intake? Do I agree with him on the need for high animal protein intake…categorically NO. (In the past, I also have felt terribly comfortable if I consumed a large red meat meal. I eat red meat at the lowest amount/frequency of my animal protein options)
Since this a rapamycin/mTOR/longevity forum, I look at nutrition (macros, quality, timing) through the lens of healthspan and lifespan. If you subscribe to the low mTOR peak/AUC = greater healthspan/lifespan (as I do), then you theoretically want to keep this signalling axis as muted as possible, whilst simultaneously maintaining other functional biomarkers, including muscle mass/strength/resiliency, namely, anti-frailty phenotype. Not eating is a not an option, but eating as little mTOR stimulating nutrients as possible and as infrequently as possible is I believe a low mTOR paradigm which I have been trying practice. Being keto, I also believe in keeping the glucose/insulin/IGF-1 AUC footprint as small as possible as a parallel longevity signal. Eating low animal protein supports this axis.
How do I n=1 balance these competing targets? The last 7 yrs has been a pivot to a strict plant fat based keto, OMAD, high daily exercise (60 mins, 45 RET, 15 zone 3 cardio) lifestyle intervention. By default, keto limits protein macros to < 20% of total calories. In these calories, I consume only highly quality wild animal protein (grass fed leanest beef cut, wild salmon, wild shrimp, sardines, eggs). After loosing 50 lbs in the first 6 months, I’ve been weight stable since, but yet have gained significant muscle mass/strength and CRF (95% percentile VO2max), all the while consuming MAX 50 grams/day of animal protein (70 kg male). I eat immediately after daily exercise to optimize MPS. The mirror and the weight scale are my muscle/calorie guardrails. How is that I have generated such massive muscle gains on such low amounts of animal protein?? Exercise physiologists say I should be eating 1g/kg/day++.
CLEARLY, resistance exercise creates a massive mechanical FUNCTIONAL hypertrophy/hyperplasia that is only PARTIALLY dependent on EXOGENOUS amino acids for growth. I’ve talked to many exercise researchers around the world about the protein/mTOR/resistance exercise axis, and it’s NOT black and white; one can build muscle with LOW protein AND blunting mTOR (with rapamycin) via RET. I was on therapeutic dose rapamycin for over 6 months (10X what anyone is taking orally), and found no functional detriments to exercise output/recovery or muscle maintenance; rapamcyin dosen’t shut everything down, there are rapamycin and mTOR independent pathways to MPS (also confirmed in private communications). Could I have larger musculature by eating more protein…likely yes, but there’s no need; I have more than sufficient strength/mass to have older age resiliency, and I further activate mTOR and compromise my ketogenic state.
What’s missing in all the sarcopenia/protein intake hype is the #1 lifestyle muscle preserving/build intervention…resistance exercise! One can overcome the inevitable decline in muscle mass/strength with age with RET without eating more protein that is “necessary”. Necessary being lean muscle mass/strength maintenance. Yes, I would lower my mTOR even further if I went strictly plant based (lower BCAA, especially leucine), but then I’d miss out on all the super nutrient profile contained in high quality animal protein. So this is where I’ve landed on the needle tip longevity balancing act for now. I tried a few plant proteins (eg. pea protein with similar and higher AA profile/content as beef so NOT lower mTOR signal on an AA basis), but extremely difficult to digest (they have intrinsically low bioavailabilty, thus lower mTOR signal I would assume, but seems futile to consume something the body cannot properly process/use), that I had to abandon. Of course, I could consume conventional plant protein foods like beans/lentils, but then I’d blow up my ketogenic homeostasis.
"Plant-based proteins have less of an anabolic effect than animal proteins due to their lower digestibility, lower essential amino acid content (especially leucine), and deficiency in other essential amino acids, such as sulfur amino acids or lysine. Thus, plant amino acids are directed toward oxidation rather than used for muscle protein synthesis"
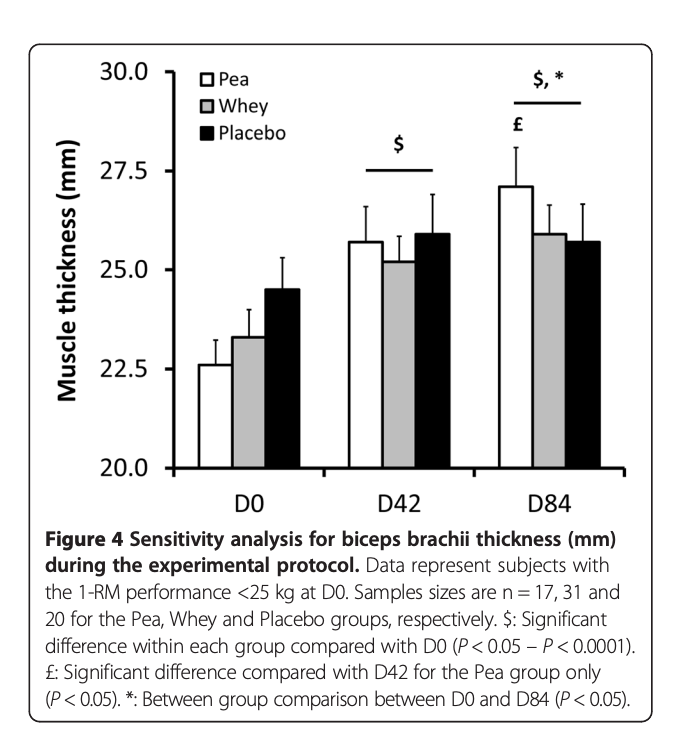
Although this large trial study found superior muscle gain results comparing WP to Pea Protein to placebo.
Maximizing Muscle Hypertrophy: A Systematic Review of Advanced Resistance Training Techniques and Methods
“From the nutrition point of view, protein intake alongside RT is a potent stimulus for muscle protein synthesis. With regard to RT, manipulation of its variables such as intensity and volume of effort, exercise order, number of performed repetitions and sets, tempo of movement, and the duration of rest periods between sets and exercises and training status have been extensively explored and discussed to maximize muscle adaptations”
As for your digestion rate questions, some literature references.
A Review of Issues of Dietary Protein Intake in Humans
https://sci-hub.se/10.1123/ijsnem.16.2.129
(I didn’t realize seafood was scientifically shown to have a far higher digestion rate than beef, but I can categorically attest to feeling nowhere near as full/satiated eating fish vs red meat)
“Much controversy exists over the advantages and disadvantages of various quantities of protein consumption and the metabolic fate of the amino acid content of various proteins, mainly due to the limited amounts of data pertaining to protein metabolism and amino acid kinetics in humans. Essentially dietary protein requirement is described as the minimum level of protein necessary to maintain short-term nitrogen balance under conditions of controlled energy intake and is quantified as the Recommended Daily Allowance (RDA) in the US. This level assumes the primary use of amino acids as substrates for synthesis of body proteins; however there is mounting evidence that additional metabolic roles for some amino acids require plasma and intracellular levels above minimum needs for protein synthesis (1). Recently a meta-analysis of 235 non-athletic individuals gathered from 19 nitrogen balance studies for estimating protein requirements in healthy adults found the median estimated average requirement (EAR), and 97.5th percentile (RDA) to be 105 mgN ∙ kg-1 ∙d-1, and 132 mgN ∙ kg-1 ∙ d-1 respectively (2). This corresponds to 0.65 and 0.83 g good quality protein ∙ kg-1 ∙ d-1, or 52 g and 66.4 g per day respectively for an 80 kg individual.”
The metabolism of dietary protein and amino acids is influenced by the composition of the specific protein, meal composition, timing of ingestion, and the amount or dose of the protein or amino acids ingested (37). The speed of absorption by the gut of amino acids derived from dietary proteins can also modulate whole body protein synthesis, breakdown, and oxidation.
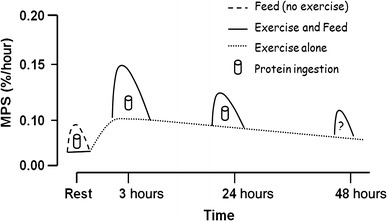
An important question then must be posed: “Does a more rapidly absorbable protein result in greater in vivo protein synthesis?” This is a central issue of large protein consumption with fitness enthusiasts, athletes, and bodybuilders. Rapidly absorbed amino acids despite stimulating greater protein synthesis, also stimulate greater amino acid oxidation, and hence results in a lower net protein gain, than slowly absorbed protein (54). LEUCINE balance, a measurable endpoint for protein balance, is indicated in Figure 1, which shows slowly absorbed amino acids (~ 6 to 7 g/h), such as CAS and 2.3 g of WP repeatedly taken orally every 20 min (RPT-WP), provide significantly BETTER protein balance than rapidly absorbed amino acids (39, 54).
The misconception in the fitness and sports industries is that rapidly absorbed protein, such as WP and AA promote better protein anabolism. As the graph shows, slowly absorbed protein such as CAS and small amounts of WP (RPT-WP) provide four and nine times more protein synthesis than WP. This “slow” and “fast” protein concept provides some clearer evidence that although human physiology may allow for rapid and increased absorption rate of amino acids, as in the case of WP (8 to 10 g/h), this fast absorption is NOT strongly correlated with a “maximal protein balance. In a study by Linn et al. (62) subjects fed a relatively high protein diet of 1.87 ± 0.26 g ∙ kg-1 ∙ d-1 over a 6-month period, consistently had elevated plasma insulin levels 8 h AFTER the last protein meal.
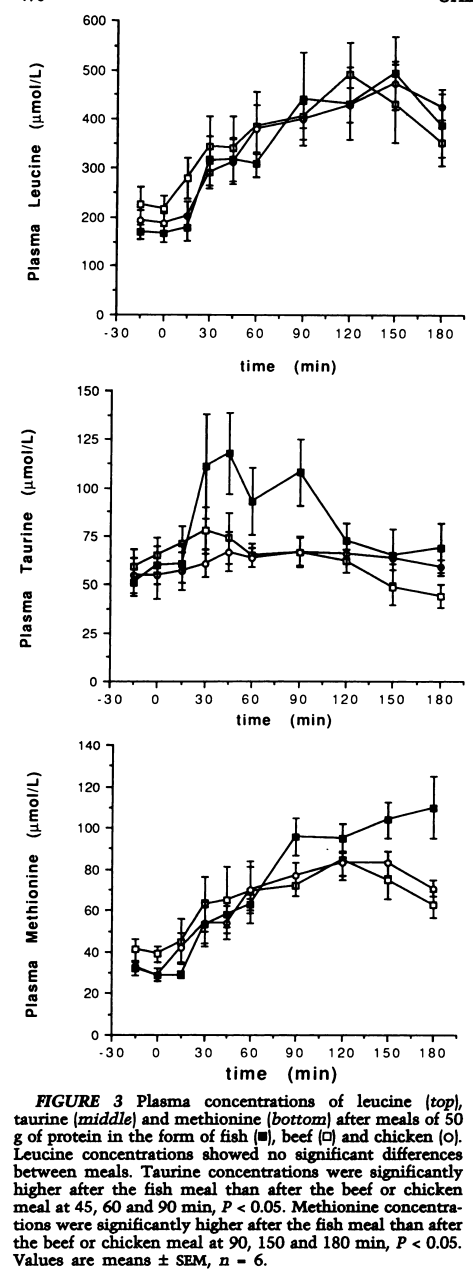
A Comparison of the Effects of Beef, Chicken and Fish Protein on Satiety and Amino Acid Profiles in Lean Male Subjects
https://sci-hub.se/10.1093/jn/122.3.467
How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution
“It has been proposed that muscle protein synthesis is maximized in young adults with an intake of ~ 20–25 g of a high-quality protein; anything above this amount is believed to be oxidized for energy or transaminated to form urea and other organic acids. However, these findings are specific to the provision of fast-digesting proteins without the addition of other macronutrients. Consumption of slower-acting protein sources, particularly when consumed in combination with other macronutrients, would delay absorption and thus conceivably enhance the utilization of the constituent amino acids. The preponderance of data indicate that while consumption of higher protein doses (> 20 g) results in greater AA oxidation, this is not the fate for all the additional ingested AAs as some are utilized for tissue-building purposes. Perhaps the most comprehensive synthesis of findings in this area has been done by Morton et al. [2], who concluded that 0.4 g/kg/meal would optimally stimulate MPS. Importantly, these estimates are based on the sole provision of a rapidly digesting protein source that would conceivably increase potential for oxidation of AA when consumed in larger boluses. It seems logical that a slower-acting protein source, particularly when consumed in combination with other macronutrients, would delay absorption and thus enhance the utilization of the constituent AA. However, the practical implications of this phenomenon remain speculative and questionable.”
“Therefore, a 40-g dose of protein equivalent to 20 g essential amino acids (EAAs)
seems to be optimal for the maximal stimulation of MPS in exercised and rested muscle of older adults”
Design:
Volunteers (n = 48) consumed a standardized, high-protein (0.54 g/kg body mass) breakfast. Three hours later, a bout of unilateral exercise (8 × 10 leg presses and leg extensions; 80% one-repetition maximum) was performed. Volunteers ingested 0, 10, 20, or 40 g whey protein isolate immediately (∼10 min) after exercise. Post absorptive rates of myofibrillar MPS and whole-body rates of phenylalanine oxidation and urea production were measured over a 4-h post drink period by continuous tracer infusion of labeled [13C6] phenylalanine and [15N2] urea.
Conclusions:
A 20-g dose of whey protein is sufficient for the maximal stimulation of post absorptive rates of myofibrillar MPS in rested and exercised muscle of ∼80-kg resistance-trained, young men. A dose of whey protein >20 g stimulates amino acid oxidation and ureagenesis"
“The “muscle full effect” concept (26) offers an explanation for why MPS rates were not augmented with 40g protein in the current and past (4) studies. This concept is based on the notion that an upper limit of amino acid delivery must be achieved before muscle cells no longer use amino acids as substrate for MPS and, instead, divert amino acids toward catabolic processes (27). Both the extracellular (25) and intracellular availability of amino acids regulates the response of MPS. Changes in EAA concentrations are sensed by a leucine membrane detector that subsequently signals the activation of mammalian target of rapamycin signaling to stimulate MPS (28)”
This paper might shed some light on your protein type/digestion rate/meal interval/mTOR activation query.
Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial
"Objective: We aimed to compare protein digestion and absorption kinetics, postprandial amino acid availability, anabolic signaling, and the subsequent myofibrillar protein synthetic response after the ingestion of milk compared with beef during recovery from resistance-type exercise
The participants performed resistance-type exercise that consisted of 4 sets of 8–10 repetitions to volitional fatigue for both the leg press and knee extension exercise (t = -60 min). After the completion of the exercise bout, a muscle biopsy sample was collected, and subsequently the participants consumed 158 g (providing 30 g protein) minced beef or an
isonitrogenous amount of nonfat skim milk (t = 0 h; Table 1)
(I’ve included a table of AA profile of various concentrated forms of proteins to compare with skim milk and beef in the embedded study)
Results: Beef protein–derived phenylalanine appeared more rapidly in circulation compared with milk ingestion (P , 0.001). The availability of phenylalanine during the 5-h post exercise period tended to be higher after beef (64% 6 3%) ingestion than after milk ingestion
(57% 6 3%; P = 0.08). Both beef and milk ingestion were followed by an increase in the phosphorylation of mammalian target of rapamycin complex 1 and 70-kDa S6 protein kinase 1 during post exercise recovery. Milk ingestion increased myofibrillar protein synthesis rates to a greater extent than did beef ingestion during the 0- to 2-h post exercise phase (P = 0.013). However, the increase in myofibrillar protein synthesis rates did NOT differ between milk and beef ingestion during the entire 0- to 5-h post exercise phase (P = 0.114).
Immediately after resistance exercise, mTORC1 phosphorylation did not differ from resting, baseline conditions. During post exercise recovery, mTORC1 phosphorylation increased at 2h (P = 0.006) and returned to baseline values at 5 h (P = 0.64), with NO differences between the milk and beef conditions (P . 0.05; Figure 5A). Here, the examination of mTORC1, 4E-BP1, p70S6K1, or rpS6 phosphorylation provided little insight into the mechanisms that govern changes in myofibrillar protein accretion after milk or beef ingestion. In the current study, we did not observe any differences in the protein phosphorylation status throughout post exercise recovery after milk compared with beef ingestion. It might be possible that the mTORC1 pathway was being activated via different mechanisms between the milk and the beef condition. For example, the higher leucinemia after beef ingestion may have been a principle driver, whereas in the milk condition, other factors (e.g., postprandial insulin response, microRNAs, or bioactive peptides) may have been responsible for activating mTORC1 signaling proteins. The reduced phosphorylation of 4E-BP1 immediately after exercise is consistent with previous observations (36) and likely represents a shift of energy provision to fuel muscle contraction rather than the synthesis of de novo myofibrillar protein (38)."
“The present study demonstrated that the ingestion of beef resulted in a more rapid release of protein-derived phenylalanine into the circulation and greater postprandial plasma amino
acid availability throughout 5 h of post exercise recovery compared with the ingestion of an isonitrogenous amount of milk. Despite the more rapid postprandial rise in plasma amino
acid availability after beef as opposed to milk ingestion, milk ingestion resulted in a greater myofibrillar protein synthetic response during the early stages of the postprandial period (0–
2 h). The overall postprandial myofibrillar protein synthetic response assessed over 5 h of postexercise recovery did not differ between milk and beef consumption. Both milk and beef ingestion augment the post exercise myofibrillar protein synthetic response in young men, with a stronger stimulation of myofibrillar protein synthesis during the early postprandial stage after milk ingestion”
In summary:
-
It appears that consuming more than say 20-40g of animal protein amino profile nutrition PER MEAL does not produce meaningful MPS gains nor overall physiological beneficial gains (nitrogen balance, urea production).
-
Although fast digestion animal protein leads to earlier bloodstream amino acid peak profile as compared to low digestion proteins such as beef, on comparable isonitrogenous intakes, both of these protein types are relatively long signalling (beef of course much longer than WP; defined as presence of amino acids in the blood stream for several hours; the time in between say normal 3 meals/day, every 4-6 hrs), and produce similar mTOR activation post intake (5h). If you ate small amounts of WP (fast digestion) with some type of time restricted feeding, you could create some no AA bloodstream gaps, and lower mTOR most certainly vs an equivalent AA bolus of red meat. However, long acting proteins can produce superior leucine balance and greater MPS gains vs fast acting. Greater leucine balance = greater mTOR activation = greater MPS. Avoiding animal protein/high BCAA foods is only way to mitigate this mTOR signal. One of the benefits of keto/OMAD is you clear out the amino acid/nutrient signal, allowing for a lower mTOR AUC over a 24 hr period, which hopefully accumulates this daily benefit over remaining lifetime.
-
Unfortunately, if you eat animal protein, be it fast or slow digestion, you activate mTOR over an extended period of time between meals. Perhaps one could transition to plant based protein or trade protein quality (lower BCAA) to lower mTOR activation, but then you compromise overall nutrient profile and MPS. But perhaps one might compensate with additional exogenous nutrients and enhanced RET. By tweaking inputs, one can navigate to maintain an acceptable homeostatic metabolic/functional state, n=1 physiologically possible.