Download PDF
Highly variable biological effects of statins on cancer, non-cancer, and stem cells in vitro
Show authors
Scientific Reports volume 14, Article number: 11830 (2024) Cite this article
Abstract
Statins, the drugs used for the treatment of hypercholesterolemia, have come into the spotlight not only as chemoadjuvants, but also as potential stem cell modulators in the context of regenerative therapy. In our study, we compared the in vitro effects of all clinically used statins on the viability of human pancreatic cancer (MiaPaCa-2) cells, non-cancerous human embryonic kidney (HEK 293) cells and adipose-derived mesenchymal stem cells (ADMSC). Additionally, the effect of statins on viability of MiaPaCa-2 and ADMSC cells spheroids was tested. Furthermore, we performed a microarray analysis on ADMSCs treated with individual statins (12 μM) and compared the importance of the effects of statins on gene expression between stem cells and pancreatic cancer cells. Concentrations of statins that significantly affected cancer cells viability (< 40 μM) did not affect stem cells viability after 24 h. Moreover, statins that didn´t affect viability of cancer cells grown in a monolayer, induce the disintegration of cancer cell spheroids. The effect of statins on gene expression was significantly less pronounced in stem cells compared to pancreatic cancer cells. In conclusion, the low efficacy of statins on non-tumor and stem cells at concentrations sufficient for cancer cells growth inhibition, support their applicability in chemoadjuvant tumor therapy.
Similar content being viewed by others
Article Open access 17 February 2022
Article Open access 16 October 2020
Article Open access 07 October 2021
Introduction
Despite significant advances in the medical science, there is still a large number of pathological conditions, such as degenerative and cancer diseases, that cannot be satisfactorily treated with standard therapies. Therefore, alternative strategies that would lead to the restoration of damaged or degenerated tissue or that would contribute as an adjuvant therapy to conventional treatment have been searched. In this sense, the application of stem cells today appears to be the most progressive therapeutic method1,2. An enormous number of studies have been published on the possibility of inducing stem cell differentiation to desired tissue types3. The most limiting factor of stem cell therapy represents the risk of disorganized cell growth, proliferation, and division that possibly lead to tumor formation4,5. One of the potential alternatives to eliminate such risk is the application of statins6.
Statins are the dominant group of compounds used for the treatment of hypercholesterolemia and cardiovascular diseases7 due to their ability to inhibit de novo cholesterol synthesis. In total, eight statins have been introduced for clinical purposes: lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, rosuvastatin, pitavastatin, and cerivastatin8. Although individual statins differ from each other in their chemical structure, physico-chemical properties, source or preparation, metabolism, etc., the common characteristic of all of them is the competitive inhibition of the rate-limiting step of the mevalonate pathway catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase9. Due to the depletion of intermediates of the mevalonate pathway, statins have, in addition to hypolipidemic effects, several other pleiotropic biological effects that play an important role in preventing the progression of many clinical conditions, including cancer diseases10.
To apply individual statins, whether, in chemoadjuvant therapy of malignant tumors or regenerative medicine, it is necessary to know in detail the mechanism of their effect on proliferation and survival of not only cancer but also non-cancerous and stem cells. Therefore, we compared in this study the effect of individual statins on the survival of stem, cancer and non-cancerous cells grown in two-dimensional (2D) and stem and cancer cells grown in three-dimensional (3D) conditions (monolayer and spheroids, respectively), and using the whole genome / whole transcriptome microarray analyses we studied biological events that were most affected by individual statins.
Results
Comparison of in vitro effects of statins on viability and growth of stem, non-cancerous and cancer cells cultured in a monolayer
Initially, non-cancerous cells HEK 293 and stem cells ADMSC were exposed to individual statins within a concentration range of 0–40 µM. This range was chosen based on the fact that IC50 values for all the statins as determined for pancreatic cancer cells MiaPaCa-2 were found to be less than 40 µM after 24 h of treatment11,12. As we did not detect any significant effect of statins on the viability and growth of ADMSC, the statin concentration range was extended to 100 µM concentration. Interestingly, even after 24 h of exposure to a statin concentration of 100 µM in cell growth medium, the number of stem cells did not decrease dramatically, except for simvastatin (Fig. 1). Simvastatin reduced the viability of ADMSC by more than 80% already at a concentration of 50 µM (p < 0.005). Compared to ADMSC, HEK 293 cells were only slightly more sensitive to the antiproliferative effect of certain statins (Fig. 1); with lovastatin, pitavastatin, and atorvastatin, the most pronounced antiproliferative effect was obtained, even at the lowest concentrations used (Fig. 1).
Figure 1
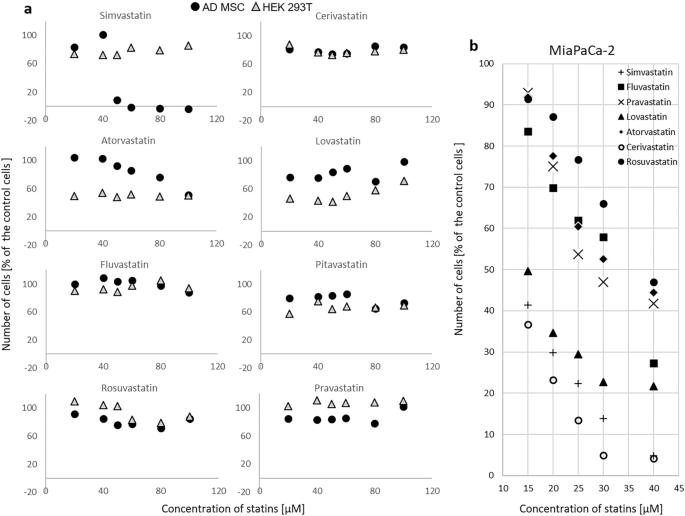
The effect of statins on viability and growth of (a) stem ADMSC and non-cancerous HEK 293 cells and (b) cancer MiaPaCa-2 cells. (a) ADMSC—human adipose-derived mesenchymal stem cells, HEK 293—human embryonic kidney cells, exposure to statins—24 h, concentrations 0—100 µM, control—methanol, (b) previously published data11, MiaPaCa-2—pancreatic cancer cells, exposure to statins—24 h, concentrations 0—40 µM, control—methanol.
Full size image
Interestingly, extending the incubation time of HEK 293 and ADMSC cells with statins to 48 and 72 h resulted in a growth inhibitory effect of statins (except for pravastatin and partially rosuvastatin), however, this effect was induced already by the lowest tested concentration of statins (20 µM), and was not significantly pronounced by increased concentration (data not shown). In contrast, in some cases (for example, cerivastatin, ADMSC, 48 h of incubation), the intensity of the antiproliferative effect of statin decreased with increasing concentration (data not shown).
The comparison of the effect of statins (20 µM) on the growth and viability of pancreatic cancer MiaPaCa-2 cells, non-cancerous HEK 293 cells, and ADMSC stem cells is shown in Fig. 2. Non-cancerous HEK 293 cells are more resistant to statins compared to pancreatic cancer MiaPaCa-2 cells even after 72 h, while a delayed effect was observed in ADMSC stem cells, similar to that observed in pancreatic cancer MiaPaCa-2 cells (Fig. 2).
Figure 2
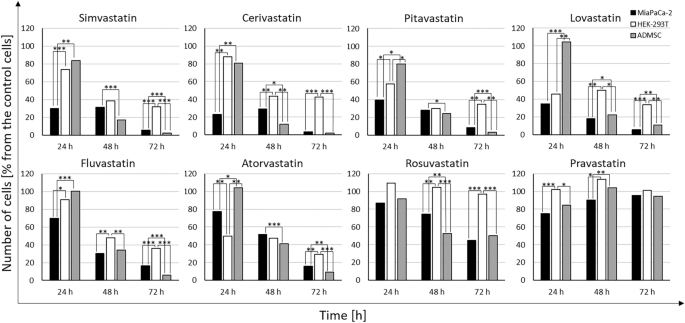
Comparison of the effect of statins on the growth and viability of pancreatic cancer MiaPaCa-2 cells, non-cancerous HEK 293 cells, and ADMSC stem cells. Concentration of statins—20 µM, Time—exposure to statins—24, 48, and 72 h, control—methanol.
Full size image
In vitro effect of statins on the size and compactness of spheroids of cancerous and stem cells
The control ADMSC spheroids formed from a small inoculum of stem cells did not significantly change their size during the nine-day observation period after the long-term cultivation (statins were added 10 and 3.5 weeks after the inoculation of ADMSC and MiaPaCa-2 cells, respectively) (Fig. 3a). However, the control spheroids of pancreatic cancer cells became observably larger during the nine-days observation period (Fig. 3b).
Figure 3
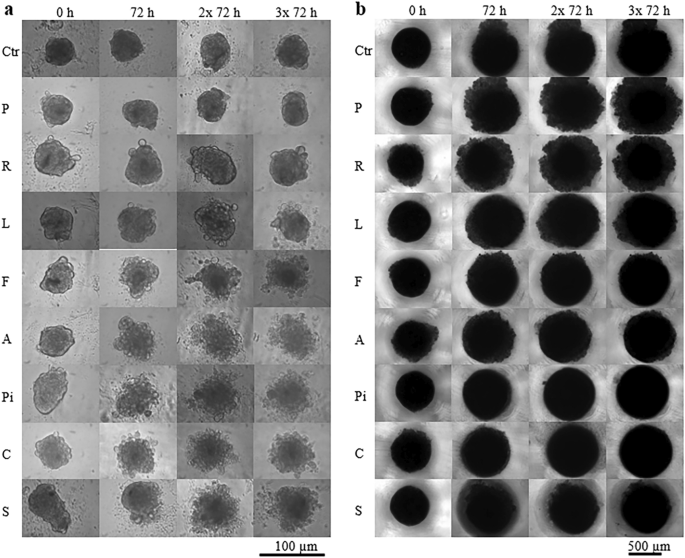
Effect of statins on size and compactness of spheroids. (a) ADMSC stem cells, (b) pancreatic cancer MiaPaCa-2 cells, concentration of statins—20 µM, Ctr—methanol treated spheroids, P—pravastatin, R—rosuvastatin, L—lovastatin, F—fluvastatin, A—atorvastatin, Pi—pitavastatin, C—cerivastatin, S—simvastatin. Statins were added once, after spheroid formation, 10 weeks (a) or 3.5 weeks (b) after inoculation. Experiment was carried out in biological dodecaplicates.
Full size image
The less effective statins (pravastatin and rosuvastatin) had no visible/detectable effect on size and morphology of the ADMSC spheroids. Spheroids affected by other statins began to decay after the first 72 h of statin treatment (Fig. 3a).
Statins, which were less effective in the 2D arrangement did not prevent the further growth of pancreatic cancer cell spheroids; however, they reduced the compactness of spheroids compared to the control spheroids (Fig. 3b, pravastatin and rosuvastatin). Other statins did not induce changes in the compactness and size of the pancreatic cancer cell spheroids when compared to the control spheroids significantly enough to be evaluated by light microscopy (Fig. 3b). Interestingly, statins that affected stem cell spheroids did not affect cancer cell spheroids and vice versa.
In vitro effect of statins on formation of spheroids of cancer and stem cells
The formation of spheroids of pancreatic cancer cells in short-time cultivation experiment (statins were added 24 h after cells inoculation) was affected by all the tested statins except for pravastatin (Fig. 4b). Only atorvastatin induced a change in stem cell spheroid formation that could be observed by light microscopy (Fig. 4a).
Figure 4
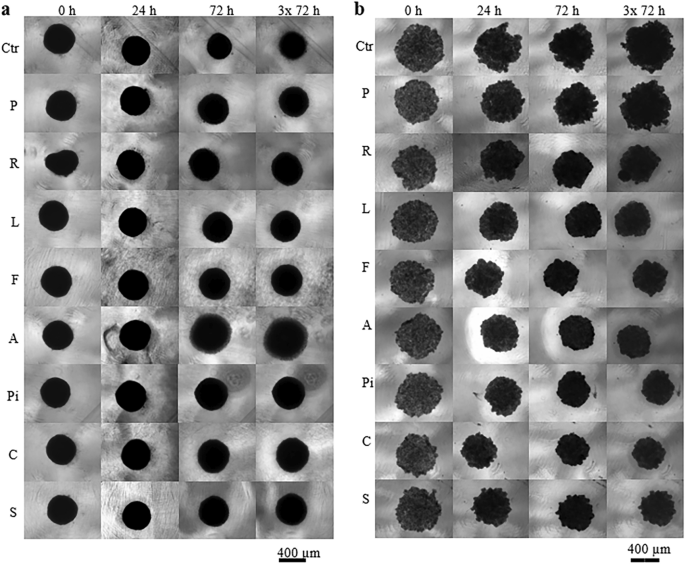
Effect of statins on the spheroid formation. (a) ADMSC stem cells, (b) pancreatic cancer MiaPaCa-2 cells, concentration of statins—20 µM, Ctr methanol treated spheroids, P—pravastatin, R—rosuvastatin, L—lovastatin, F—fluvastatin, A—atorvastatin, Pi—pitavastatin, C—cerivastatin, S—simvastatin. Statins were added once, 24 h after cell inoculation. Experiment was carried out in biological dodecaplicates.
Full size image
Comparison of in vitro effects of statins on 2D and 3D experimental cancer and stem cells models
The effect of statins on the formation of spheroids from MiaPaCa-2 cells corelated with the effect of statins on the viability of cells growing in monolayers. Pravastatin (20 µM) had no effect on viability of MiaPaCa-2 cancer cells growing in a monolayer (Fig. 2) as well as on formation of spheroids (Fig. 4b). All other statins reduced the size of MiaPaCa-2 spheroids (Fig. 4b), that corelated with the inhibitory effect of statins on viability of MiaPaCa-2 cancer cells growing in a monolayer (Fig. 2).
We observed a delayed effect of statins (20 µM) on ADMSC stem cells grown in a monolayer (Fig. 2), only pravastatin was not effective. However, statins did not affect the formation of ADMSC spheroids; the only exception was atorvastatin, whose action resulted in the magnification of spheroids compared to the control ones (Fig. 4a).
The effect of statins on the size and compactness pre-formed MiaPaCa-2 cell spheroids was exactly opposite to their effect on the viability of cells grown in a monolayer. Interestingly, statins that were ineffective or only slightly effective in the 2D arrangement (pravastatin, rosuvastatin, atorvastatin, fluvastatin) (Fig. 2) had the most visible effect on the compactness of the spheroids (Fig. 3b). On the contrary, the effect of statins on the size and compactness of pre-formed spheroids of ADMSC stem cells (Fig. 3a) correlated well with the effect of statins on the viability of ADMSC stem cells grown in a monolayer (Fig. 2). In both experimental ADMSC models (2D and 3D), all the statins, except for rosuvastatin and pravastatin, were effective.
Comparison of the in vitro effect of statins on the gene expression of pancreatic cancer MiaPaCa-2 cells and ADMSCs stem cells.
The transcriptional microarray analysis was used to study the effect of statins on the gene expression of ADMSC stem cells cultured for 24 h in 2D conditions in the presence of statins. Statins were administered at a concentration of 12 µM, which corresponds to the simvastatin IC50 value for MiaPaCa-2 cells after 24 h, used in the study of the effect of statins on gene expression of MiaPaCa-2 cells11.
Pravastatin-treated cells had the same transcription profile as control cells. Other statins significantly changed the transcription profile compared to that of control cells, of which rosuvastatin had the weakest effect (repository number E-MTAB-11579).
Comparison of the effect of statins on the gene expression of pancreatic cancer MiaPaCa-2 cells and ADMSCs stem cells is shown in Fig. 5.
Figure 5
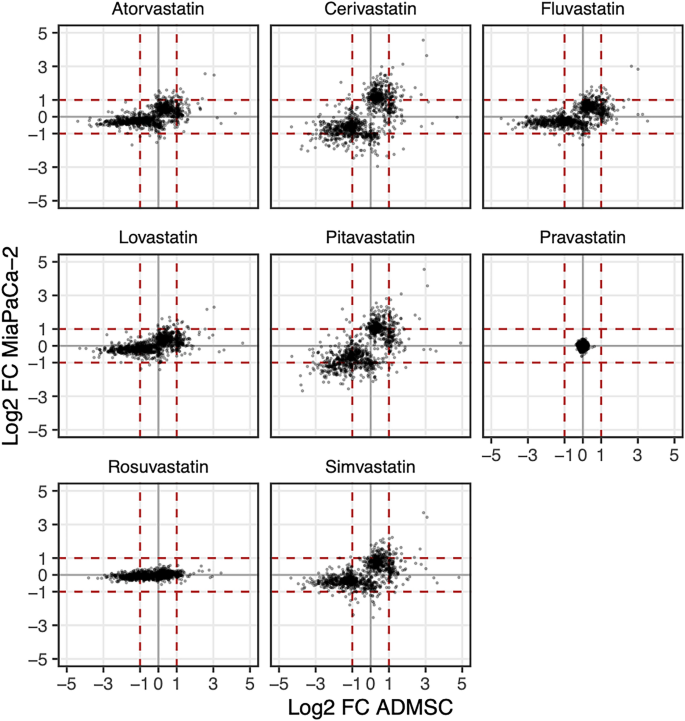
Comparison of expression changes between statin treated and control MiaPaCa-2 and ADMSC cells. Displayed are only the genes that are differentially expressed upon at least one statin treatment in at least one cell type, requiring |log2FC|> 1 and FDR < 0.05. Statins were administered at a concentration of 12 µM for 24 h. (FC fold change, FDR false discovery rate, horizontal and vertical axes—changes in ADMSC and MiaPaCa-2 cells, respectively, upon respective treatment). The red dashed lines indicate two-fold change increase or decrease in the gene expression. The genes with at least two-fold up-regulation (resp. down-regulation) in ADMSC stem cells are displayed to the right (resp. left) of the dashed lines. Similarly, genes with at least two-fold up-regulation (resp. down-regulation) in cancer cells are displayed above (resp. below) of the dashed lines. For details about differentially regulated transcripts see the ArrayExpress database, accessions E-MTAB-3979, E-MTAB-11579.
Full size image
In general, the trend of the effect of statins on the gene expression related to their lipophilicity is conserved in both cancer and stem cells12, specifically, the gene expression increased gradually with the lipophilicity of statins. The exception was the effect of rosuvastatin: rosuvastatin did not affect the gene expression of pancreatic cancer cells, while its effect on the transcription of stem cell genes was statistically significant (Fig. 5). Statins induced more frequently the gene down-regulation than up-regulation in stem cells, the trend was opposite in cancer cells (Fig. 5).
Comparison of the most significantly affected cellular pathways (according to KEGG) induced by statins in the cell lines studied is shown in Fig. 6.
Figure 6

Cellular pathways most significantly affected by statins in cancer and stem cells. The gene set enrichment analysis (GSEA) revealed the KEGG pathways most affected by statin treatment in ADMSC and MiaPaCa-2 cells. Displayed is the union of the top five most enriched pathways among the comparisons. (Statin concentration—12 µM, treatment time—24 h, p-value—GSEA p-value, gene ratio—fraction of KEGG pathway genes among differentially expressed genes). For details about differentially regulated transcripts, see the ArrayExpress database, accessions E-MTAB-3979, E-MTAB-11579.
Full size image
In stem cells, statins significantly affected the cell cycle (Fig. 7a), especially decreased the expression of genes encoding several cyclin-dependent kinases (E-MTAB-11579). Significant effect of statins on DNA replication (Fig. 7b) is related to the decrease of transcription of genes encoding the components of the PCNA complex, DNA polymerases α, δ, ε, helicase and DNA ligase, that indicates cell cycle arrest in S phase (E-MTAB-11579).
Figure 7
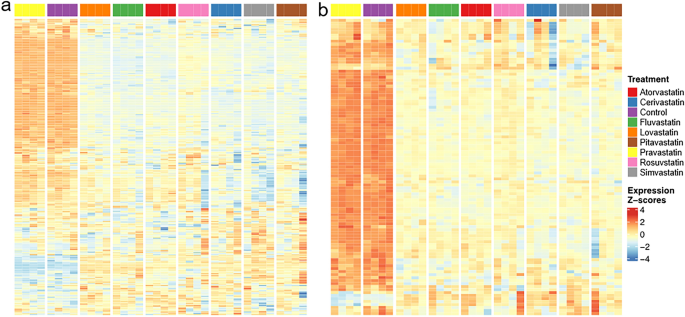
The effect of statins on (a) cell cycle, (b) DNA replication in ADMSCs. Figure represents heatmaps of z-score of the log expression intensities of differentially expressed genes. Presented are only the genes with statistically significant difference (FDR < 0.05) in expression intensity in at least one comparison statin vs. control and at least two-fold change of the expression intensity after the statin exposure. ADMSD—human adipose-derived mesenchymal stem cells, concentration of statins—12 µM, treatment time—24 h, the gene sets are based on KEGG pathways hsa04110 (cell cycle) and hsa03030 (DNA replication). For full list of differentially regulated transcripts see the ArrayExpress database, accession E-MTAB-11579.
Full size image
Discussion
Although the antiproliferative effect of statins on various cancers has been reported in a large number of studies, the effect of statins on stem cell viability has been published in only a few articles13,14,15,16,17,18. Furthermore, in most studies, the properties of all statins available on the market have not been collectively studied under identical conditions. The current paper and our previous reports11,12,19,20 are based on the evaluation of statins using the same experimental model under the same conditions. Statins in concentrations corresponding to those achieved with daily doses of statins in the treatment of cardiovascular diseases (lovastatin 20–80 mg, simvastatin 10–40 mg, fluvastatin 20–80 mg, pravastatin 10–80 mg, atorvastatin 10–80 mg, rosuvastatin 5–40 mg21) were used in our in vitro assays. Such concentrated solutions of statins (5–40 μM) with proven anti-proliferative effect on pancreatic cancer cells in vitro after 24 h of exposure11 did not affect the proliferation of the HEK 293 non-cancerous cells and ADMSC stem cells. We were unable to observe any effect of statins on stem cells even at a concentration of 100 μM after a time period of 24 h. Similar concentrations (30–60 μM) were used in study by Izadpanah et al., where they observed a significant increase in the doubling time of mesenchymal stem cells exposed to pravastatin and atorvastatin during the initial two passages22.
Some statins (e.g. lovastatin) belong to substances whose IC50 values decrease significantly by lowering the pH from 7.5–7.7 to 6.7–6.823,24. Therefore, in chemoadjuvant therapy of specific types of tumors characterized by local pH reduction, statins could be used even at lower doses than in hypercholesterolemia treatment. Therefore, the antiproliferative or pleiotropic effects of statins on non-cancerous and stem cells would be minimal.
Studies investigating changes in gene expression induced by statin treatment using microarray technology have been published since 200025,26. These results are difficult to compare due to the usage of different statins, different statin concentrations, or experimental models. Moreover, in the case of the array analysis, the results are difficult to compare even between our experiments. Although we used the same tested concentration of all statins for both ADMSCs and pancreatic cancer cells (12 μM, 24 h), the microarray platform was changed between experiments. As the probes design and sensitivity of platforms differ, the absolute number of genes whose expression was affected, as well as the intensity of changes in the expression of individual genes is not entirely comparable. However, it is obvious that the trend in the effectivity of statins on the gene expression related to their lipophilicity is preserved both in tumor and stem cells12. The only exception is rosuvastatin, which was ineffective at the concentration tested in pancreatic cancer cells; however, its effect on stem cell gene transcription was statistically significant (Fig. 5).
Atorvastatin, simvastatin, fluvastatin, lovastatin, cerivastatin, and pitavastatin are relatively lipophilic, while rosuvastatin and pravastatin are hydrophilic. While hydrophilic statins cannot easily pass through the cell membranes and are taken up mainly by hepatocytes, which are also the target of statin treatment from a therapeutic point of view, lipophilic statins, due to their easier passage through cell membranes, also reach other types of cells27. The question is whether rosuvastatin enters stem cells more easily compared to tumor cells in general or only to adipose tissue derived stem cells